most sophisticated industrial inorganic chemistry:
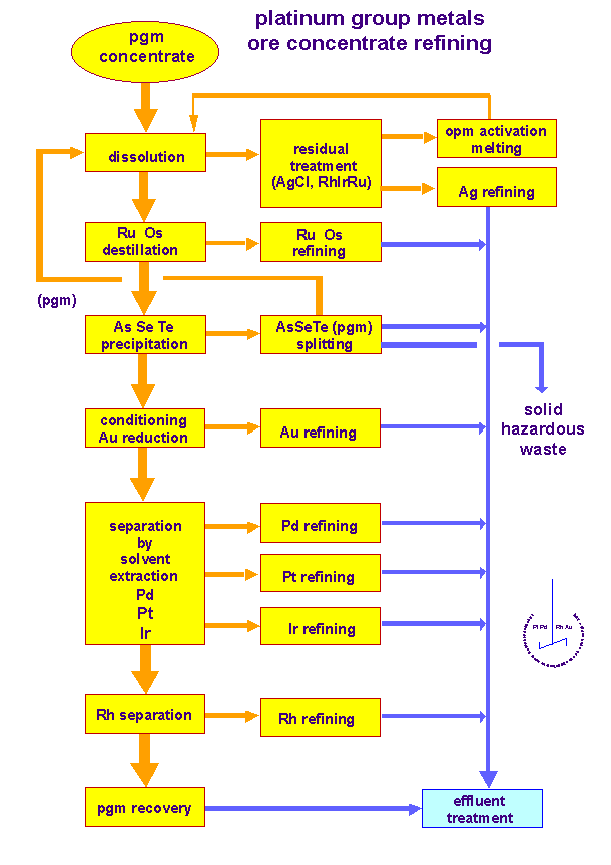
refining of platinum group metals ore concentrate

pgm ore
concentrate
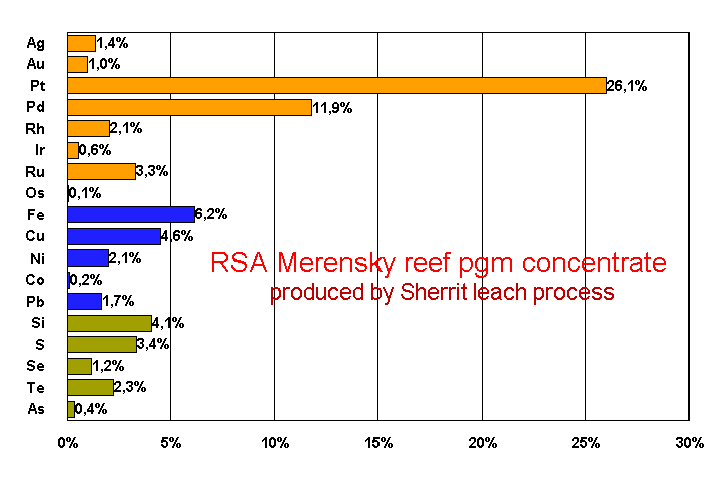
The figure below shows a typical assay of a South
African platinum group metals ore concentrate obtained as the filter
cake after sulfuric acid leach (according to the Sherrit process) of
a Merensky reef converter matte.
In fact the Sherrit process
results in two completely different behaving concentrates which are
blended normally by concentrate producers for fast value recovery.
The larger fraction (L) is resulting as a direct residue of multiple
pressure leach, the minor one (M) from the recovery of rhodium
dissolved during acid leach.
More attention should be paid in the
future to completely avoid fraction M.
The total precious
metal content of the concentrate is between 40 and 50%. The higher
the precious metal content in the feed the less reactive is the
concentrate (which is bad for pgm refining but saves some transport
cost for those refiners who send their materials overseas).
dissolution
The ore concentrate is dissolved in hydrochloric acid applying
appropriate oxidants. Other acids than hydrochloric acid are not
applicable in technical plants due to environmental control of the
process.
Normally more than 98% of Pt Pd and Au, more 90% of
Rh, Ir and Ru should be dissolved during dissolution; Ag is left
undissolved as AgCl. But due to mistreatment of Sherrit fraction M
the dissolved fraction may decrease to 20% Rh, 50% Pd, 80% Pt and
less than 10% Ru resulting in a dramatic reduction of first pass
yield and cash lock-up in a pgm refinery.
residual
treatment and opm activation
The filter cake from dissolution
mainly consists of AgCl, SiO2, Cr2O3 and opm oxides. After optional
separation of SiO2 (in case of high quantities) silver is separated
and passed to the silver refining line. Concerning value silver is of
neglectable importance in a pgm refining factory; of course it is
recommended not to invest in a Ag refining line just because of these
small quantities in pgm concentrates.
The remaining opm rich
material is fed to a special melting process in which opm's are
activated again for a second acid leach.
Ru Os
distillation
The presence of ruthenium interferes the quality
of palladium, platinum, iridium solvent extraction and rhodium
precipitation. The same is true for classical old fashioned more
pollutive process routes based on precipitation of Pd, Pt, and Ir as
their low soluble hexachloro complex compounds. Therefore in pgm
refinery circuits Ru is separated as early as possible below 50 ppm.
Hence the filtrate after dissolution is passed to the
separation of ruthenium and osmium by distillation and reactive
absorbtion; most refiners don't keep trace of the later. The feed may
be processed in the same equipment as the leach step (under
approppriate conditions). Special attentencion has to be paid for
design and construction materials of this process because the
volatile Ru compound might cause explosions and the volatile Os
compound is very poisonous.
The distillate/absorbate itself
is passed to the ruthenium refining line comprising again
distillative equipment for the separation of Os from the bulk of
ruthenium under appropriate conditions.
As Se Te Fe
separation
The pregnant solution after Ru Os separation is
further treated to remove As Se Te Fe by precipitation. This process
is necessary only for high amounts of these elements, not necessarily
for the ore concentrate mentioned above. This combined removal of
impurities cares for
- elemination of harmful components (As) in
the final waste water volume
- deminishing of components which
may prolongate rhodium recovery and purification in the rhodium
refining line (Se Te)
- separation of elements blocking or
reducing the capacity of platinum solvent extraction (Fe)
The
disadvantage of this process is that some Pt and Pd is precipitated
too if this process is not controlled carefully. Therefore eventually
the separated solid has to be treated to recover a small percentage
of platinum and palladium locked in this cycle.
In total this
process step means a considerable upgrade in purity of the pregnant
liquor with positive influence on first pass yield of Pd, Pt, Rh and
recovery time of rhodium
gold
reduction
From the filtrate of (hazardous) metal waste
removal gold is separated by reductive precipitation. The quality of
the primarily separated material is >99.5% (good delivery). One
more dissolution (aqua regia etc.) and reduction will yield gold
>99.95% up to >99.99% (fine gold) especially if Se and Te have
been removed thoroughly in the step before.
It is possible to
remove the gold from the main stream by solvent extraction too (HEV
CHEMICALS
is supplying extractants for this process) but it is not recommended
in the pgm ore concentrate refining cycle because the amount of gold
is quite low; hence this process would be rather cost-ineffective.
palladium
solvent extraction
From the filtrate of gold precipitation
after adjusting appropriate feed conditions palladium is separated by
solvent extraction applying DnHS or DnOS as the extractant (HEV
CHEMICALS
is supplying the extractants for this process). Old-fashioned
ineffective refineries still use the traditional much more pollutive
precipitation process entraining for instance large amounts of
ammonia or other pollutants into the estuary.
It depends upon
the scale of operation whether the solvent extraction plant is
designed as a continuous or batch process. In either case the
complete solvent extraction process comprises
- extraction;
palladium is transferred into the organic phase
- scrubbing;
reduction/removal of coextracted impurities from the extract
-
stripping; transfer of Pd back into a second pure aqueous phase for
palladium recovery and further processing
- solvent make-up;
cleaning the organic before extraction again
The purity of
palladium in the strip solution is about 99%, main impurities are
silver and platinum. The process is very effective in the rejection
of non-precious metals.
platinum,
iridium solvent extraction
Similar like palladium, platinum
and iridium are separated from the raffinate of the palladium
extraction. Of course the extractants, conditions and equipments
differ. For low concentrations of iridium it is not recommended to
extract the metal form the main stream. In this case it is more
efficient to concentrate the main stream before iridium extraction or
separate iridium from platinum within the platinum extraction cycle
or further downstream in the platinum refining line.
The
purity of the platinum leaving the solvent extraction cycles is about
99.5%. Main impurities are iridium, Se and As.
rhodium
precipitation
Rhodium is separated from the raffinate of the
platinum or iridium extraction by precipitation applying a highly
selective organic under appropriate conditions.
The purity of
the dissolved rhodium precipitate is >99%, main impurities are
iridium and bismuth.
platinum
group metals trace recovery
Finally traces of platinum group
metals being present in the barren liquor from time to time on a
level of 5 - 10 ppm are recovered by electrolytic cementation and
sulfide precipitation if necessary.